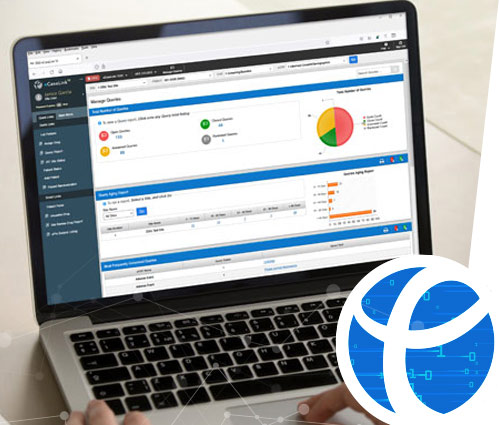
The industry's most robust reporting capabilities integrated within eCaseLink.
DSG's software applications offer over 65 standard reporting tools ' at no additional cost. In addition, our ad hoc reporting tool is the most comprehensive available. Create up to 200 unique reports based on criteria you select. Since all data captured by the system is clean and in real time, access to your critical data is just a click away.
DSG offers eight standard privilege levels, so access to reports remains secure. Data cannot be changed, modified or reviewed unless the user has specific rights to the reporting tools. As with all of our applications, DSG's reporting tools are fully integrated and available via the web on a standard Internet connection. You may also access reports using our powerful standalone tool, CaseXportTM, making sure wherever you are, you can review up-to-the-minute clinical data.