eCaseLink eConsent

eConsent a convenient and efficient informed consent tool
Secure, flexible, and convenient data capture
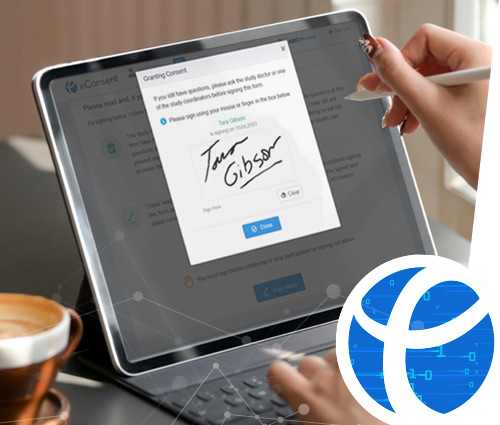
eCaseLink eConsent can be used as a standalone or apart of the fully harmonized unified eCaseLink EDC platform to capture participant data conveniently on-site or off-site using any web-connected devices such as smartphones, iPads, mini tablets, laptops and desktops. The eConsent system provides all users a secure responsive patient-centric design, that enables participants to view study related documentation and grant esignature consent to take part in a clinical trial saving valuable time at study sites.
Flexible and convenient data capture
eCaseLink eConsent is a simple intuitive tool designed for real-time, real-site, anywhere data collection situations, such as in operating rooms, clinics, or within a doctor's office or fully remote. The application ensures that participant consent time/date and signature is accurately and unambiguously recorded, along with investigator signature, to eliminate the potential of review findings.
Multimedia information
eCaseLink eConsent provides study information to potential study participants in written, visual and audio-visual formats, to enable optimal information exchange.
eCaseLink eConsent features:
- More efficient and effective for participant understanding of content
- Presents information in multi-media fashion and not linear on paper is more effective
- Real-time data collection and TeleHealth support
- Built in eSignature functionality
- Promotes remote monitoring, improving data quality and participant engagement
- Supports interactive videos and other validated assessment tools