Comprehensive, easy-to-use secure clinical trial management software
We offer the most comprehensive, easy-to-use clinical trial management software for any CRO or sponsor in the pharmaceutical, medical device and life sciences industries.

We offer the most comprehensive, easy-to-use clinical trial management software for any CRO or sponsor in the pharmaceutical, medical device and life sciences industries.
We spearheaded the move from paper-based documentation methods to the most effective, efficient online tools and digital resources. Our EDC software is optimized for any clinical trial, academic research or any other research study.
Since our founding, DSG has transformed clinical trial data collection and analysis with its integrated suite of electronic data capture software products and solutions for faster ROI and more successful FDA submissions.

Our user-friendly interface gives you complete control to build your study the way you want it, providing flexibility for every type of study.

Offers a user friendly, efficient platform for data entry and data analytics.

Allowing data entry provides a secure, flexible, and convenient approach data capture.

A streamline fully integrated randomization and trial supply management system with eCaseLink platform.

Say goodbye to paper surveys, make it easier for patients to complete questionnaires.

Optimize the Patient Experience & Lower the Barrier to Enrollment.
Meet your enrollment goals with a comprehensive platform to track pre-screened participants.
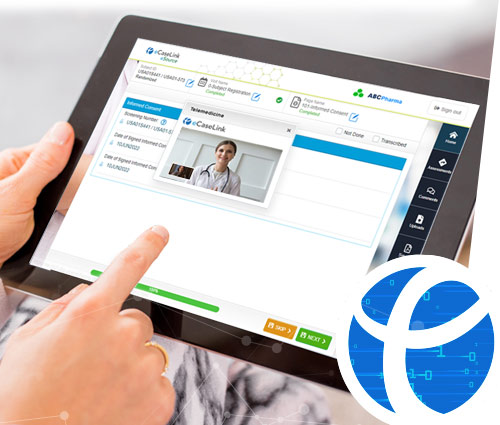
Empower your sites and investigators to communicate with participants anywhere.
Easy tool for Pharmacovigilance to keep track of Safety events and helps report to regulatory bodies.

Provides unparalleled control over your study with advanced reporting, protocol deviations, workflow events, and more.