eCaseLink eSource
Want to learn more?
Download PDF
Want to learn more?
Download PDFProvides a secure, flexible, and convenient approach to on-site or off-site Participant data capture. eSource can be used as either a standalone or a harmonized EDC platform. With our responsive patient-centric design, participants may enter data to answer eCRFs conveniently from any Wi-Fi-enabled location using any browser or web-enabled device.
eSource is an electronic data capture (EDC) tool designed for real-time, real-site, anywhere data collection situations, such as in operating rooms, clinics, or within a doctor’s office. Everything is designed to make the participant survey experience simple, fast and intuitive with no need for any additional training.
eSource enables your clinical trial personnel the freedom and flexibility to enter clinical data anyplace-anytime removing the need for source data verification and dramatically decreasing monitoring time and costs. eSource is built for all web-connected devices including iPads, mini tablets, smartphones and desktops.
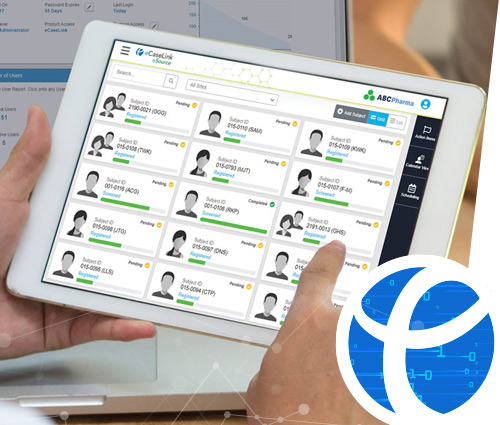
eSource is a patient-centric data collection tool that encourages real-time data entry during the Participant's visit. Entering Participant data in real-time eliminates unnecessary duplication of data and reduces the possibility of transcription errors.
Using eSource allows Investigators to better engage with Participants, satisfying visit scheduling and other needs.
The simple intuitive user interface is a fast and convenient way to enter source data in real-time, more accurately and all within the time-frame of the Participant's visit.
eSource can be accessed in any location using any type of web browser on any Wi-Fi-enabled devices such as tablets, mobile phones, laptops or desktops.